The SH3 Domain Acts as a Scaffold for the N-Terminal Intrinsically Disordered Regions of c-Src
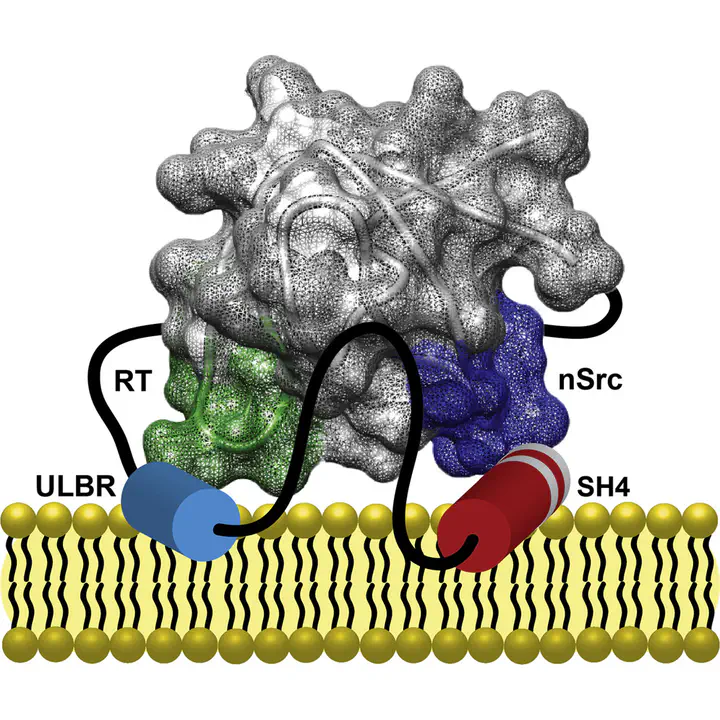 Image by M. Maffei. Elsevier user license
Image by M. Maffei. Elsevier user licenseAbstract
Regulation of c-Src activity by the intrinsically disordered Unique domain has recently been demonstrated. However, its connection with the classical regulatory mechanisms is still missing. Here we show that the Unique domain is part of a long loop closed by the interaction of the SH4 and SH3 domains. The conformational freedom of the Unique domain is further restricted through direct contacts with SH3 that are allosterically modulated by binding of a poly-proline ligand in the presence and in the absence of lipids. Our results highlight the scaffolding role of the SH3 domain for the c-Src N-terminal intrinsically disordered regions and suggest a connection between the regulatory mechanisms involving the SH3 and Unique domains.
Type
Publication
In Structure