 Image by M. Arbesú. CC BY
Image by M. Arbesú. CC BYAbstract
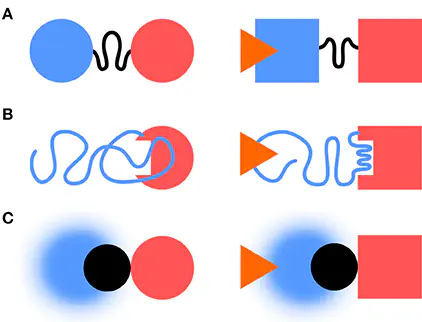
Structural disorder is an essential ingredient for function in many proteins and protein complexes. Fuzzy complexes describe the many instances where disorder is maintained as a critical element of protein interactions. In this minireview we discuss how intramolecular fuzzy interactions function in signaling complexes. Focussing on the Src family of kinases, we argue that the intrinsically disordered domains that are unique for each of the family members and display a clear fingerprint of long range interactions in Src, might have critical roles as functional sensor or effectors and mediate allosteric communication via fuzzy interactions. A large majority of proteins are build from domains, classically defined as functional autonomous folding units. In a typical divide-and-conquer approach, the structure-function analysis proceeds through the characterization of the individual domains followed by the study of their mutual interactions. This approach makes a clear distinction between the “functional” domains and the linkers separating them. The same strategy is taken in the analysis of the also very abundant multiprotein complexes, in which the individual proteins are considered the building blocks (equivalent to domains of multidomain proteins). Key components of multiprotein complexes are scaffolding proteins, which would play the role of linkers in multidomain proteins. The current view of protein-protein interactions is quite dynamic and intrinsically disordered regions (IDR) are increasingly recognized as key players. In this minireview we shall summarize some important aspects of (intermolecular) protein binding by disordered proteins and extend them to the case of interdomain (i.e., intramolecular) binding using the c-Src family of kinases as an example.